An Ebola vaccine trial that was conducted in Guinea in 2015, reveal reports by the World Health Organisation and the medical journal The Lancet.
The vaccine, called rVSV-ZEBOV, was studied in a trial involving 11,841 people in Guinea during 2015.
Out of the 5837 people who received the vaccine, no Ebola cases were recorded 10 days or more after vaccination. In comparison, there were 23 cases 10 days or more after vaccination among those who did not receive the vaccine.
The trial was led by WHO, together with Guinea’s Ministry of Health, Medecins sans Frontieres and the Norwegian Institute of Public Health, in collaboration with other international partners.
“While these compelling results come too late for those who lost their lives during West Africa’s Ebola epidemic, they show that when the next Ebola outbreak hits, we will not be defenceless,” said Dr Marie-Paule Kieny, WHO’s Assistant Director-General for Health Systems and Innovation, and the study’s lead author.
Experts say the latest findings are a major step towards the vaccine getting approval. In fact, the vaccine’s manufacturer, Merck, Sharpe & Dohme, this year received Breakthrough Therapy Designation from the United States Food and Drug Administration and PRIME status from the European Medicines Agency, enabling faster regulatory review of the vaccine once it is submitted.
In addition, GAVI, the Vaccine Alliance provided US$5 million to Merck towards the future procurement of the vaccine once it is approved, pre-qualified and recommended by WHO. As part of this agreement, Merck committed to ensure that 300 000 doses of the vaccine are available for emergency use in the interim, and to submit the vaccine for licensure by the end of 2017. Merck has also submitted the vaccine to WHO’s Emergency Use and Assessment Listing procedure, a mechanism through which experimental vaccines, medicines and diagnostics can be made available for use prior to formal licensure.
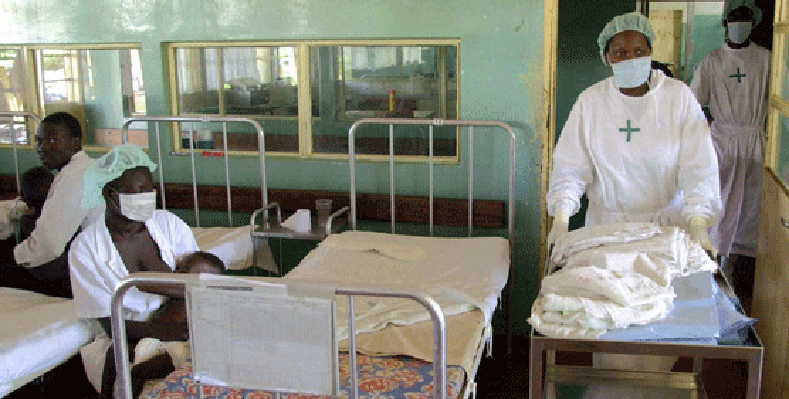
Since Ebola virus was first identified in 1976, sporadic outbreaks have been reported in Africa. But the 2013–2016 West African Ebola outbreak, which resulted in more than 11 300 deaths, highlighted the need for a vaccine.
The findings are also further evidence of the power of modern biotechnology, especially Genetic Engineering in finding solutions to deadly diseases in humans, animals and plants.
According to WHO, the vaccine was developed by replacing a gene from a harmless virus known as vesicular stomatitis virus (VSV) with a gene encoding an Ebola virus surface protein. The vaccine does not contain any live Ebola virus. Earlier trials have shown the vaccine to be protective in animals, and be safe and produce an immune response in humans.
Studies are ongoing to provide more data on the safety of the vaccine in children and other vulnerable populations such as people with HIV. In case of Ebola flare-ups prior to approval, access to the vaccine is being made available through a procedure called “compassionate use” that enables use of the vaccine after informed consent. Merck and WHO’s partners are working to compile data to support license applications.
The rapid development of rVSV-EBOV contributed to the development of WHO’s R&D Blueprint, a global strategy to fast-track the development of effective tests, vaccines and medicines during epidemics.